We asked several HIT vendor executives the following question: What was the biggest HIT-related news or event in 2011 and why?

Ray Dyer, CEO, Acusis
The industry has experienced continued mergers in 2011 (Allscripts/Eclipsys and Medquist/M*Modal, to mention a couple), the federal government has come to a ruling on how ACOs should work, MU payments were issued, and we have seen a rise in the use of and demand for mobile devices in healthcare, including new medical students being issued iPads.
I don’t think a singular event could be identified as the biggest moment in HIT in 2011. However if mapped across a timeline, we could certainly mark these as significant highlights during this past year.

Dan Herman, Founder and Managing Principal, Aspen Advisors
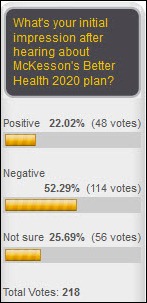
McKesson Provider Technology (MPT) division’s Better Health 2020 strategy announced on 12/9/11. MPT has publicly announced what has been rumored for some time – that it will curtail further development of its Horizon software product line and invest heavily in its Paragon product. This decision affects hundreds of hospitals throughout the country. Some long-term McKesson customers have already seen the writing on the wall and have made strategic and financially significant decisions to move away from McKesson’s non-integrated Horizon clinical platform. However, many other customers (medium-sized community hospitals and multi-entity integrated delivery networks) are faced with a critical decision: do we stay the course with McKesson or rethink our EMR strategy and pursue an alternative course?
Reading between the lines, this change in direction will have a huge financial impact on current McKesson Horizon customers, and it brings McKesson’s credibility in the EMR market into question. Although the market for EMR technologies has grown, the Horizon product line has experienced decline in market share, system development delays, poor product integration, and significant customer dissatisfaction.
On one hand, I laud MPT for coming clean on the challenges it has had with the development and support of the Horizon product. However, it appears that MPT’s go forward strategy is “déjà vu” – a poorly thought out approach to integrate disparate platforms, enhance a product that has experienced success in a focused market place (Paragon), and promise customers that MPT is committed to delivering “a fully integrated core clinical and revenue cycle IT system.”
Regardless of McKesson’s direction, healthcare providers need to evaluate their needs against the software available to support them and make decisions that are in their best interests.

Jay Deady, CEO, Awarepoint Corporation
The biggest HIT-related news of 2011 is that 1,211 eligible hospitals and 21,425 eligible professionals (EPs) have successfully attested and collected $1.84 billion from Medicare and Medicaid through the end of November for meeting Stage 1 meaningful use of electronic health records (EHRs).
The news, disclosed at the December 7, 2011 meeting of the Health IT Policy Committee, is significant for several reasons. First, it indicates the transformation of the health system and national commitment to improve access, quality, coordination, and efficiency of care through information technology have begun in earnest.
Second, it ensures providers adhere to baseline quality standards that can be measured and compared, including: using CPOE for medication orders, implementing decision support rules, exchanging key clinical information, and protecting electronic health information.
Third, attesting hospitals and EPs had to meet reporting requirements that some providers have characterized as challenging, onerous and time-consuming. Those that haven’t attested or plan to attest to Stage 1 in 2012 will benefit from lessons learned by early attesters.
Fourth, the payments demonstrate the meaningful use program is real, which will motivate providers to automate sooner rather than later in order to subsidize or recoup the cost of an EHR. As they automate, however, providers must remember that EHRs alone won’t address every problem afflicting healthcare. To accomplish that and aid throughput, they must complement EHRs with other technologies such as real-time location systems.

Don Graham, General Manager, Billian’s HealthDATA
I would have to say it’s been the extreme focus providers seem to be putting now on enterprise healthcare analytics and business intelligence, especially when coupled with the recent release of final ACO regulations. They realize that healthcare transformation requires more than the implementation of an EMR. As providers move more towards the coordinated care model (PCMH, ACOs and beyond), the challenge will be to meet the analytical needs to support this model. It will be critical to deliver an integrated view of clinical, financial and operational data not only across the enterprise, but across the community as well. Access to this data will enable providers to move into new areas of care.
In acknowledgement of the importance of analytics and its role in healthcare transformation, Billian’s HealthDATA and affiliate Porter Research have decided to focus one of our Provider Perception studies on Enterprise Analytics early next year. It’s something that our healthcare vendor customers are in turn paying more and more attention to, and appreciate the insight into.

Stuart Long, President, Capsule
I think the biggest HIT-related news this year is ARRA. It seems that all hospitals are just looking at how they are going to meet the criteria and qualify for the stimulus dollars. They are looking for any and all news on the topic. They are looking for help in sorting through and interpreting the legislation and what they specifically need to do to qualify. They are setting up cross-functional teams within the hospital to sort through it all. And they are looking to their vendor partners to prove how their technology and solutions fit within ARRA and therefore whether or not they will make it to the hospitals short list or not.
In fact, it’s one of the biggest areas we’ve been working on at Capsule and with our EMR partners — to help define where device integration fits within meaningful use and CPOE. I believe that ARRA and CPOE have hit all areas of the HIT space, from hospitals, to vendors, to system integrators, and to the publishing community at large – whether that be new publications, journals, trade shows, blogs, and even social media.
I just believe that the entire healthcare IT world has been turned upside down with ARRA. And of course it’s not over. We will all continue to be focused on it as Phases 2 and 3 roll out in the years to come. It might be a bumpy ride, but all we can do is hold on tight and keep on moving forward.

Mac McMillan, CEO, CynergisTek
I’m sure from an overall HIT perspective the answers will be quite different, but from a privacy and security perspective, it had to be one of two announcements from the federal government.
The first is OCR announcing it will finally initiate its regular audit program to conduct routine, random compliance audits of organizations accountable to HIPAA. The initiation of the audit program completed OCR’s menu of compliance and enforcement-related activities by adding to its existing practices: investigation of breaches and response to complaints. The OCR will likely disclose more information about these audits and their outcomes next year.
The second candidate for the biggest HIT-related event is the ONC announcing a delay in Meaningful Use Stage 2 in order to give organizations more time to meet Stage 1 requirements and to further support adoption of EHR technologies.
Both of these have a direct impact on the industry and its move to privacy and security readiness. We at CynergisTek are working with a lot of healthcare organizations to achieve compliance goals and improve their overall security posture. We’ve seen Meaningful Use increase attention to security and work to provide the support covered entities need to accomplish their risk analysis and implement the security features of their EHR/EMR effectively. This approach is consistent with OCR’s guidance on risk analysis and produces a step-by-step action plan that guides remediation efforts. In addition to that, we understand the partnership model desired in healthcare and we provide ongoing advice and assistance throughout the remediation process and beyond.

Michael O’Neil, CEO, GetWellNetwork
2011 was the year that the healthcare industry fully recognized patient engagement as a business imperative. Driven generally by the reality of a shift to a value-based US health system — and specifically by the requirements for patient engagement in Stage 2 Meaningful Use and the push by ONC to support consumer health IT adoption — leading providers moved aggressively to develop strategies, find partners, and adopt solutions to help.
Due to the confluence of regulatory, business, and technology transformation underway, patient engagement emerged as a core strategy for performance improvement for providers. Leveraging HIT, patient engagement solutions can take the form of Web-based portals and interactive patient care (IPC) education and communication solutions, or as simple as an online patient guide or patient bill of rights. It’s education. It’s entertainment. It’s empowerment. In fact, it’s all aspects of being involved in care.
One thing is sure: providers must work to ensure that the principle of patient engagement permeates clinical and business workflow and every aspect of care delivery. In 2012, there is no doubt that a focus on patient engagement will take greater hold throughout the continuum of care.

Peter J. Butler, President and CEO, Hayes Management Consulting
Attempting to identify the biggest HIT-related news item is difficult. However, based on research from the Ponemon Institute, which conducted detailed interviews with executives at 72 healthcare organizations, the economic impact of information breaches can be far reaching and should be considered one of the top news items. Consider the following breach survey results:
- On average, organizations surveyed have had four data breach incidents in the past two years, up from three in last year’s study.
- The average number of lost or stolen records per breach is 2,575, up from 1,769 in the previous study.
- The top three causes for a data breach are lost or stolen computing devices, third-party mistakes, and unintentional employee action.
- Insufficient budgets and inadequate risk assessments are cited as the two greatest breach prevention weaknesses.
- Some 81% of those surveyed use mobile devices to collect, store, or transmit patient information, but 49% say they’re doing nothing to protect these devices.
There hasn’t been a slow news day in healthcare this year, but the topic of breaches is the alarm bell for the industry. With the onslaught of new technology, the most important data – patient health information – is not safely secured. It reflects the cracks in the frantic attempt to catch up with all the unintended byproducts of the industry changes.
For example, just when the industry secured all its laptops, mobile technology took off. Now, smart phones, mobile carts, tablets, and thumb drives need to be monitored and secured. Not a day goes by where a laptop has been lost or stolen. HIPAA’s reporting policy means that the clock is ticking when the breach happens, organizations are responsible for business associates’ breaches, and the fines pale in comparison to the impact on reputation.
Healthcare organizations must put privacy and security at the top of their 2012 priority list. They must develop policies and procedures for reporting in a timely manner on breaches. Further assessments need to be conducted on a timely basis – annually at least. This protocol must be understood by every employee and monitored to ensure compliance across the enterprise. Training and communication of HIPAA regulations need to incorporated within each healthcare organization and monitored. Micky Tripathi’s blog post on this topic gives a stunning insider view to what it’s like to have a breach happen in your organization. No one is immune – and it takes a village to rectify it.

Tiffany Crenshaw, President and CEO, Intellect Resources
Meaningful Use and ICD-10 are what everyone is talking about, but surprisingly, no one is talking about what Meaningful Use and ICD-10 mean from a human resources standpoint. Incentive deadlines have created an industry-wide shortage of experienced healthcare IT professionals, specifically those with Epic experience. Think about it — everyone in the industry is working towards the exact same deadline!
Countless planning hours are put forth to ensure a successful go-live and meet incentive deadlines. Technology, equipment, infrastructure, training and other task related functions are all meticulously planned in advance with the intention of those tasks being carried out by future experienced healthcare IT hires. But what if you can’t find those future hires? What happens to your plan then?
Meaningful Use and ICD-10 have created a huge demand for experienced healthcare IT professionals and there simply aren’t enough experienced individuals to fill these much-needed positions. Now is the time to think creatively about hiring solutions and start planning.

Doug Burgum, President and CEO, Intelligent InSites
The biggest healthcare IT event in 2011 was the Department of Veterans Affairs’ decision to improve healthcare efficiency with a national investment in Real-Time Location Systems (RTLS.)
The VA has the nation’s largest integrated healthcare system: over 150 medical centers, almost 1,400 community outpatient clinics, and 53,000 independent licensed healthcare practitioners, serving more than 8.3 million Veterans every year. Eric K. Shinseki, Secretary of the VA, has identified 16 transformational initiatives for the VA. One of these initiatives, Health Care Efficiency, directly includes RTLS to improve care while lowering costs.
The Government Executive reports that the VA views a planned $550 million National RTLS implementation as a way to improve efficiency, track equipment, and properly sterilize medical instruments.
We are thrilled that our industry has the opportunity to contribute to this groundbreaking effort. Intelligent InSites’ customers have improved patient satisfaction and patient safety, while decreasing hard-dollar expenses, through their use of RTLS solutions for asset management and patient flow. They do this by automatically collecting real-time data from throughout the hospital, processing that data, then using actionable intelligence to make fundamental improvements to how they run their hospitals. By using RTLS, they can process the location and status of every critical piece of medical equipment, patient, and staff in a healthcare facility, then use this data and intelligent workflow automation to improve their healthcare processes.
The next few years are going to be amazing—not just the RTLS industry, but also for the transformational benefits to the delivery of care.
Mike Sweeney, President and CEO, maxIT Healthcare

This was such a busy year in the HIT industry, it would be difficult to name just one event, so I will limit myself to three.
First, Epic’s continued dominance in new account sales along with the recent trend of their customers’ rolling the Epic applications out to their affiliate networks (acute and ambulatory) has had a major impact this year. Second, McKesson’s announcement of their product direction with Horizon and Paragon will have a significant ripple effect in the market as we get into 2012. Finally, the government mandates and programs surrounding Meaningful Use, ICD-10 and ACOs are driving so much demand and activity in the market, those can’t be left out of top events for 2011.
It’s definitely an exciting time to be in our industry, and I can’t wait to see what’s in store next year.

Tom Carson, CEO and President, MD-IT
The clear emergence of physician preferences for mobile applications to interface with their HIT systems has huge implications for systems criteria and future workflow demands. Most legacy HIT systems were developed with the idea that physicians would sit in front of desktop computers for their documentation and care-planning tasks. After years of physician reluctance to adapt to these defined work processes, the relatively rapid increase in popularity of applications for smart phones and tablets suggest that doctors are beginning to participate on their terms in EMR adoption and use, and that HIT vendors are due for a re-think in their approach to product solution design and delivery.
This is significant because it marks a maturing of the EMR industry in that products will have to be more customer-driven going forward, as opposed to regulatory-driven to succeed.

Patrick Hampson, Chairman and CEO, MED3OOO
The biggest news related event was just recently announced by the Kathleen Sebelius, the HHS secretary. By relaxing the time frame for meeting Meaningful Use Stage 2 requirements, physicians, EHR vendors, HIE projects, and most all operators of healthcare will all get more time to adapt to the rapid expansion of electronic health care delivery.
Changing healthcare to a more seamless, interconnected health system in the US is absolutely critical. Doing it right and not just doing it fast is even more critical. Why have providers go from a paper mess to an electronic mess? Why just add technologies without the strategy, knowledge, and planning needed and the provider level knowledge necessary to actually get a return on your investment? How about a return in care for your patient?
Physicians and hospitals were forced to scramble to adopt new work flows, new processes of care delivery, and at the same time, implement new technologies (EHR systems) and install patient portals to meet the requirements for Stage 1. If these same providers want to stay viable, they have to maintain productivity, fight competitive forces in their markets and pre-pay, for most of this while the government gives them an IOU. Throw in the current economic conditions of our depressed economy and it is all too much for providers. Great for vendors, not so great for providers.
Relaxation of the Stage 2 requirements deadline is less about not wanting to get somewhere, but it is more about getting there efficiently. A pet peeve of mine is why have financial penalties should you not do it fast? Why not financial incentives for doing it right?
Hopefully we will all take this delay as a continued opportunity to improve the designs of technology, not just to process a bill or serve as expensive transcription, but provide technology that can really support providers and the coordination of care across the populations they serve. While Accountable Care is touted as the next big step for many, MED3OOO as a healthcare management and technology company has provided technologies and operations for physicians and hospitals and communities are already accountable for care and for managing risk and been doing it successfully for more than 15 years.
For those that haven’t, the learning curve and the costs are steep. These organizations will require more than just production systems like accounting systems, practice management systems, and electronic health records. Organizations will require and we will provide strategies, systems, and operations economically. Organizations will need to have capabilities that include integration of disparate systems and disparate centers of data. Organizations will need the ability and systems to disseminate population data and then distribute that same data efficiently back to the point of care for its best use.
MED3OOO is uniquely position to partner with providers and provide our existing packages of system, software, and knowledge infrastructure to make this next journey a successful one for new Accountable Care providers. In doing this, we will continue our mission and make absolutely sure that the patient has a better outcome. We do believe that outcomes matter!

Peter Kuhn, CEO, MEDSEEK
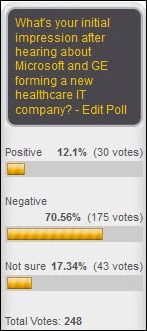
With Microsoft partnering with GE, Google exiting the PHR market, and more payers entering the provider market, the events of 2011 have significantly changed the healthcare industry. How patients respond to payers acquiring providers depends on whether they perceive an improvement in care coordination or feel that quality of care is mitigated by payers influencing physicians. With access to both risk models and clinical information, how well these acquisitions work also depends upon the ability to analyze that data to create effective business strategies.
Analytics, particularly predictive analytics, has been in the healthcare news this year. At MEDSEEK, we understand that analyzing data and creating actionable strategic plans will help our clients set themselves apart. That’s why we made the decision to acquire Third Wave Research, Ltd. We’ve subsequently integrated what we believe to be the most advanced predictive analytics technology into our strategic patient engagement and management solutions. With the commoditization of patient portals and patient outcomes and wellness driving revenues, the major market differentiator will be how well hospitals engage new and existing patients, manage costs, and coordinate care.
Hospitals’ success will increasingly hinge on using predictive analytics to match high-value patients with appropriate, profitable services when and where they need them and make informed investment decisions based on population health propensities. Combined with factors such as demographic, financial, and insurance data, predictive analytics will play a larger role in executing cost-effective, personalized marketing campaigns that influence patient behavior and improve population health.

Jeff Surges, CEO, Merge Healthcare
With new federal policies to reduce reimbursement and foster information sharing, the question is not if, but when a healthcare system should implement an enterprise imaging strategy that focuses on providing electronic access to medical images. Hospital CEOs, CIOs and physicians can no longer ignore:
- Patient safety issues around radiation exposure
- Competition to attract and retain the best physicians
- Compliance with Meaningful Use guidelines
- Support for an ACO strategy
- The push for interoperability in EHRs across healthcare systems and HIEs
These issues are driving the need for a new paradigm of image sharing and enterprise-wide imaging strategies.
An enterprise imaging strategy must focus on image storage, the ability to ingest images from outside of the enterprise, and provide accessibility to any type of medical image, anywhere across the continuum of care, anytime, by anyone. It should include three components:
- Storage. A vendor-neutral archive (VNA) to create a patient-centric record of images across all sites and modalities.
- Gateway. An intelligent DICOM gateway, capable of receiving and morphing studies from outside the enterprise.
- Viewer. A universal viewer that can be used in any environment on any device and is accessed via the browser, thus requiring no software to be downloaded.
There really is no wrong way to proceed with an enterprise imaging strategy as long as decision-makers fully understand the capabilities of each technology component, relate the capabilities to meeting their top business challenges, and look at the enterprise imaging strategy as a solution that can grow and evolve over time.

Jay Mason, CEO, My Health DIRECT
I see the roll-out and clarification for Accountable Care Organizations this year as being a significant event or turning point. While there is yet to be much to determined in how they will play a role in reimbursement and delivery, it has forced organizations — both payers and providers — to rethink how they function and how they look at themselves. Infrastructure, resources, and relationships will be reshaped.
This means they must commit to new ways to work together as providers, as well as how they are engaging, informing, managing, and directing patients. The silos that have been built up over the past decades will soon come crashing down.
Janet Dillione, Executive Vice President and General Manager, Nuance Healthcare

ICD-10 came onto the scene in 2011 in a big way. It received enormous attention throughout the year and has even been compared to Y2K. In short, it’s a monumental shift from what is documented, coded, and billed against today in healthcare and what level of specificity will be expected to be documented, coded, and billed against come 2013.
As a result of this major transition in medical coding, the reimbursement process will get more complicated and will expand. At Nuance, we believe that ICD-10 success begins with the physician at the point of documentation. In turn, over the past year we’ve allocated resources to develop, in partnership with 3M, next generation ICD-10-ready clinical documentation and coding solutions. In 2012, we’ll bring to market Computer-Assisted Physician Documentation, a new category of solutions that will interact with physicians at the point of documentation to review and prompt for the level of specificity required to achieve reimbursement in correlation with ICD-10 standards. Simply put, it turns clinical language (doctor’s spoken words) into codable language.
By combining Nuance’s speech recognition and Clinical Language Understanding (CLU) capabilities with 3M’s experience in developing and implementing coding products and services around the world, we’re well positioned to deliver solutions that will substantially improve physician workflow and will facilitate more precise, complete coding, and more accurate claims.

Todd Cozzens, CEO, Accountable Care Solutions, Optum
This past year has been filled with game-changers – from Meaningful Use to ICD-10 – but the biggest milestone in healthcare IT had to be the CMS final rule on the Medicare Shared Savings Program, which I believe will be looked back at one day as the event that spawned a whole new generation of health information technology.
Notice that I’ve not include the “care” ending to health. This next generation of IT solutions will focus not just on patients currently seeing doctors and being admitted to hospitals. It will cover a much broader spectrum of wellness and prevention products to comprehensive management of patient populations. The final regs set the tone for the industry and made a statement: accountable care is here to stay.
When I talk about accountable care these days, I am quick to point out I’m talking about the general idea of healthcare providers taking on some form of risk – i.e. being responsible for the outcome AND the cost of that outcome. McKinsey thinks there will be around 750 MSSP ACOs within five years. That sounds about right. But when we at Optum talk about the broader concept of sustainable health communities, we believe that every health system in the country will adopt some form of risk taking – whether it be bundled payments or new risk-sharing health plans with payors, all the way up to fully capitated networks.
Healthcare reform was the catalyst that started the dialogue. The budget deficit, with its inevitable cuts in entitlements, created the sense of urgency. The Medicare MSSP program signaled that CMS is 100% behind the effort. We all know that when CMS coughs, the US healthcare industry has a cold, and that’s why this final rule is such a game changer.

Paul Brient, President and CEO, PatientKeeper Inc.
One of the biggest HIT-related news stories in 2011 was the extension in the effective date of Stage 2 Meaningful Use. At some levels, this was a non-event. ONC’s decision to push back the Stage 2 start date for Stage 1 “early attesters” by a year (to Oct. 1, 2013) corrected a flaw in the original plan that effectively penalized hospitals for being on the ball and attesting for Stage 1 early. Yet the move created a fair amount of confusion in the market and headlines in industry media, and paused some Meaningful Use activity.
That is largely behind us now. The Stage 2 proposed rule will be published in the next month or so, and many hospitals are beginning to focus on the challenge of getting widespread physician adoption of CPOE and documentation software that will be required by Stage 2 and 3 objectives.

Todd Johnson, President, Salar
In a conference this past summer with chief medical information officers and physician informaticists, a physician’s panel was convened to consider the next big challenge for their hospitals. Without question, the conversion to ICD-10 topped the list. As discussed in that conference in Ojai, California, and paraphrased here: “ICD-10 is entirely a physician documentation issue.” While this statement does not to suggest a minimized importance for consultative services, back-end billing systems, or payer reform, it does call to light the critical nature of a physician’s clinical note. All coding starts from the note. Indeed, the note itself justifies all other pieces in this puzzle.
Going into 2011, we believed (as did many others) that Meaningful Use would continue to dominate all discussions. In retrospect, we’ve been surprised to see how quickly ICD-10 has become a front-burner issue though so poorly addressed by the healthcare market. Both hospitals and the vendor community alike seem woefully ill-prepared to address this issue which simply fuels the anxiety and paranoia of this “great unknown.” Just as Meaningful Use taglines blanketed the banners at HIMSS this year and MS-DRG coding dominated the banners in years before, you won’t be able to walk 10 paces without seeing ICD-10 lingo at HIMSS 2012.
Stephen Hau, CEO, Shareable Ink

The growing acceptance of enterprise cloud computing in healthcare has been a persistent and recurring theme of 2011. We are excited by this development because it will help accelerate much needed innovation in healthcare IT.
Hugely successful IT projects in our industry are relatively rare. Some may feel that this reputation is unfair and not deserved. However, who can deny that the industry has a track record of protracted implementation projects, lengthy development / release cycles, and expensive hardware investments that can quickly become obsolete?
Enterprise cloud computing represents an approach that is compelling for small physician practices (with no in-house IT support), large health systems (that require scalability, minimal administrative burden, and stringent uptime commitments), and organizations in between. We have observed shorter deployments, transparent and non-disruptive updates, and removal of hardware obligations from the customer. A cloud-based distribution model allows software providers to respond to market demands more nimbly – and healthcare organizations to take advantage of rapid innovation.
Taken together, enterprise cloud computing has the power to provide a very positive IT experience, allowing healthcare organizations to focus on the benefits of technology and creating a better environment for innovation.
Ed Daihl, CEO, Surgical Information Systems

Hospitals and anesthesia providers are facing reimbursement changes, shifting payment models, increased regulatory reporting, and Meaningful Use. We believe that hospitals will continue to focus on achieving ARRA funding to help combat these challenges.
It is important to us at SIS to ensure that we help our clients respond and prepare for these changes. We were the first perioperative healthcare software provider to achieve ONC Meaningful Use certification in 2010, and more recently, our Anesthesia Information Management System (AIMS) achieved Meaningful Use certification as well.

Evan Steele, CEO, SRS
One of the biggest HIT-related events was the recent announcement by HHS that it was postponing the implementation of Meaningful Use Stage 2. It was important, not because it will directly and significantly impact very many providers, but rather because of what it acknowledges and the positive message it sends.
All that the delay does is to reward early adopters by giving them access to three years’ of incentives under the easier rules of Stage 1 and to remove one of the reasons that might have discouraged some of them from attesting in 2011. Contrary to Secretary Sebelius’s contention, it can do nothing to speed adoption since the announcement came too late to impact any providers who have not already implemented—and been successfully using—a certified EHR.
What the delay does accomplish, however, is to send a heretofore unheard message—one of flexibility and of an acknowledgment of the significant challenges that Stage 1 is posing for many providers and vendors. By delaying the schedule for Stage 2, the government has let them know that the rules are not set in stone, that some things are negotiable, and that it is willing to work with providers and vendors in the interest of accelerating EHR adoption. If this same message is conveyed in the release of the actual rules for Stage 2, the likelihood of the program’s ultimate success will be greatly enhanced.
Rick Stockell, President, Stockell Healthcare Systems

As we have seen and experienced with our customers, the impact of prepping for and addressing Meaningful Use and ICD-10/5010 compliance on the healthcare industry was certainly significant in 2011, and it will continue to be a major factor into 2012 and beyond.

Richard Atkin, President and CEO, Sunquest
Over the past several years, structured reporting of laboratory results has been promoted as a public health priority. Its inclusion as a Meaningful Use objective is helping to drive the accelerated adoption of EMRs.
This past January, Sunquest Information Systems became the first and only dedicated laboratory information system vendor to achieve 2011/2012 Meaningful Use compliance and certification as an EHR Module by the Certification Commission for Health Information Technology (CCHIT.)
Sunquest continues to lead the industry with regard to the HITECH Act and Meaningful Use regulations. Meeting with government officials and continuously monitoring client preparedness are just two of the ways that Sunquest helps its clients complete the three steps for earning incentives: adopt certified electronic health record (EHR) technologies, achieve Meaningful Use objectives, and apply for incentive payments.
In addition to meeting many of the Meaningful Use modules, Sunquest solutions provide the capability to improve the overall safety, quality, and efficiency of healthcare. Our comprehensive Meaningful Use plan includes expanding the use and management of LOINC and SNOMED-CT codes. This initiative is a critical priority for our clients as they plan their LOINC strategy.
Sunny Sanyal, CEO, T-System

The recent decision by the US Department of Health and Human Services (HHS) to delay by one year the start date of Stage 2 Meaningful Use of certified electronic health records (EHRs) is by far the biggest HIT-related development of 2011.
By pushing the compliance deadline from Oct. 1, 2013 to Oct. 1, 2014, HHS recognized that the original timetable was too aggressive. Many hospitals planning to be ready for Stage 1 in 2011 or 2012 would have faced little lead time to prepare for Stage 2 requirements, which will not be finalized until July 2012. This deadline would have made it extremely difficult for both facilities and vendors to upgrade and install Stage 2-compliant EHRs by October 2013.
Prior to the delay announcement, we were beginning to hear rumblings from hospitals about potentially abandoning efforts to qualify for Meaningful Use incentives, instead waiting to attest until 2015 to avoid Medicare reimbursement cuts. The one-year grace period will enable providers who attest to Stage 1 Meaningful Use in 2011 to qualify for three payment years and those attesting in 2012 two payment years. It will give them and their EHR vendor partners additional time to develop a plan for Stage 2 compliance and design and implement optimal software. More importantly, it buys hospitals time to drive adoption of EHRs intended to improve the quality, safety and cost-effectiveness of care.






























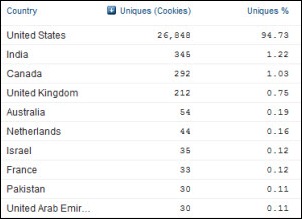

































































































I initially questioned the profile's authenticity because all of the headshots in the profile are clearly generated or enhanced by…