An HIT Moment with … Marc Andiel, CEO, Accent on Integration
An HIT Moment with ... is a quick interview with someone we find interesting. Marc C. Andiel is co-founder, president, and CEO of Accent on Integration of Murphy, TX.
What integration-related parts of Meaningful Use Stage 2 will the average hospital struggle to meet?
With Meaningful Use Stage 2, hospitals and providers are under more pressure than ever to demonstrate the use of CPOE, record and chart vital signs changes, and effectively leverage clinical decision support. In this environment, it’s imperative that healthcare organizations make the automatic acquisition of device data a reality. It saves significant time, streamlines documentation processes, facilitates valid and accurate orders, ensures clinicians have the most recent and relevant patient data, and reduces errors.
In fact, we’re seeing that clinicians are outright demanding this automation. But because patient care device interfacing requires considerable time, effort, and resources, many providers simply cannot support the effort.
One significant struggle is that in most hospitals, medical devices have historically been completely separated from the information technology group. They may reside on proprietary networks, as well as closed, non-interoperable deployments. Breaking medical device data out of these silos is imperative to meeting the integration-related Meaningful Use Stage 2 core measures.
Manufacturers began addressing this problem by providing modality-specific solutions. This model worked at first, but it resulted in many one-off projects that didn’t benefit the organization as a whole. But with the onset of Meaningful Use, providers made it a priority to take a more enterprise approach. We’re seeing that more than ever, provider organizations are refusing vendor-specific integration offerings and instead demanding enterprise-wide, vendor-neutral solutions like our Accelero Connect integration platform to interconnect a multitude of disparate technology systems.
Organizations will continue to struggle with integration projects unless they deploy solutions that are architected to facilitate the convergence of medical device technology and information technology. Additionally, caregivers, IT, biomedical / clinical engineering, and vendors must come together and take a patient-centric approach to fully unite people, processes and technology.
How many hospitals have integrated their medical devices with their clinical IT systems and what lessons have they learned in doing so?
From our experience, basic level vital signs device integration with clinical IT systems is the exception, not the rule. Far more hospitals have this on their roadmap than the number of facilities that have already completed basic vital signs integration.
It’s important to note that there is a huge gap when it comes to full medical device integration with clinical IT systems like monitors, smart pumps, ventilators, glucometers, and smart beds. Hospitals that have integrated medical and patient care devices with their clinical systems are finding that many devices beyond monitors will send clinical parameters that are not supported by their clinical systems.
Because basic vital signs integration for monitors — bedside, continuous feed, low acuity — is still uncommon for most hospitals, the real challenge that lies ahead is connecting more complex devices that will require clinical support of several more parameters.
Quite a few companies offer medical device integration products and services. How is Accent on Integration different?
Our software-only solution has zero requirements to be at the point of care. Another difference is that we don’t see ourselves as simply a product company. We will always function as both a services and a product company because we believe this will result in the most benefit for our customers. This is extremely important to us because the services component of our business allows us to be very in tune with what device manufacturers are doing now and with their product roadmaps.
It also means that we stay well informed of the current capabilities of consuming systems — like EHR, BI/CDS, EDIS, etc. — and most important, we remain in touch with clinical workflow and everyday clinician realities and challenges. To us, without an intimate knowledge of the devices, the IT systems, and the end-users’ needs, it is highly unlikely that a product alone can meet its envisioned purpose.
In addition, we routinely work for the big healthcare IT and medical device vendors to integrate their systems. We feel that the breadth of our knowledge of the different systems available in the market today and how they work is unsurpassed by any competitor. Lastly, we have extensive experience working for and with provider organizations, clinical IT vendors, RHIOs, HIEs, and technology companies.
Your leadership team all worked for Baylor. What made you decide to start a company and what’s good and bad about working for yourselves?
Jeff McGeath and I started AOI in 2006 with a simple vision that there has to be an easier way for healthcare organizations to connect their disparate systems. We reflected on our expertise and recognized that although we were very proficient in the IT system integration space, the future of healthcare relied on connecting disparate devices that housed an incredible amount of clinically critical information. Additionally, it was becoming more and more necessary for providers to be able to exchange information outside of the walls of their organization.
There was so much change and flux at the time that we weren’t completely certain the industry would go in the direction we predicted. As with most startups, things didn’t come together overnight. However, eventually we were providing services for device manufacturers as well as for one of the first HIE vendors.
Eventually it became clear that our early predictions and focus areas were growing into very important healthcare verticals. We are proud to have been a key player in steering the path of integration for the last six-plus years. Because we forged early roots in this space, today we are able to say that AOI can provide services from the device to the connected community and everything in between. We can offer expert services to providers, hospitals, and vendors alike.
While we always knew we wanted to be both a services and products company, we absolutely wanted to make sure there was a need. A benefit of working without outside influences like investors and private equity is that you have complete control of the focus of the company. You can be much more nimble. Certainly there is the early, day-to-day struggle of bootstrapping the organization. However, seven years later, we are much better for it and have been able to take the needed time to evaluate the market.
As we built our organization’s capabilities and grew our services offerings, we were able to keep a keen eye on where there were market gaps we were interested in. We were able to easily work toward filling those gaps.
One of the hard parts about working for ourselves has been building our team so that we can provide the level of professional capabilities we offer today. Finding exceptional people is hard and very time-consuming work. In our previous jobs, we were fortunate to work with great individuals who were already in place, but when we started Accent on Integration, we had to start from scratch and build a team of professionals that we knew would contribute to the company’s success.
Everyone has a core group of people that they have worked with in the past that, if given the chance, they would want them by their side again. You pointed out that our leadership team all has previous ties, and Jeff and I would have it no other way. We did everything we could to bring those folks on board, and it is a continuous process to add to our team of all-stars. Our employees are our greatest asset.
What new integration needs do you see developing for hospitals in the future?
Full waveform integration is definitely a hot topic with hospitals today. Every customer we meet with has questions about the best way to get waveform data out of their ancillary systems and into the hospital EHR in a format that can be viewed natively. Today, this is sometimes accomplished by attaching documents or scanning strip images. But what we’re seeing is that hospitals are pushing the EMR vendors toward native support of this rich data.
The market has matured to the point that basic HL7 interchange is not really a challenge for hospitals and vendors using a variety of tools. Richer content — such as waveforms and CCDA — and the orchestration of multi-step technical workflows to support clinical workflows are the integration needs we see the industry heading toward. The standards organizations like HL7 and IHE are already a few years into that stage of integration readiness, with one example being IHE’s Waveform Content Management (WCM) profile.
We also expect to see EHRs supporting a richer set of parameters from devices so a greater amount of device data can be integrated. As more data is available in real-time, alerting will continue to mature, which will greatly improve patient care and safety and has the potential to significantly improve overall operations.
In addition, we see HIEs and ACOs having community-based offerings that leverage device data not only from the hospital, but also from any location including the home.
Lastly, the interface engine market appears to be experiencing some redistribution, and there will be provider organizations that will need people skilled in both product X and product Y to do a good migration of interfaces.




















































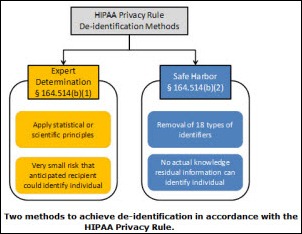


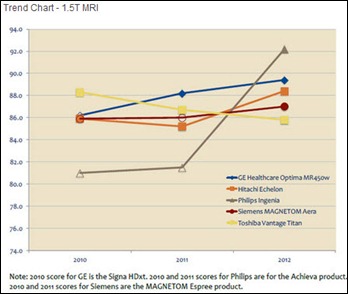
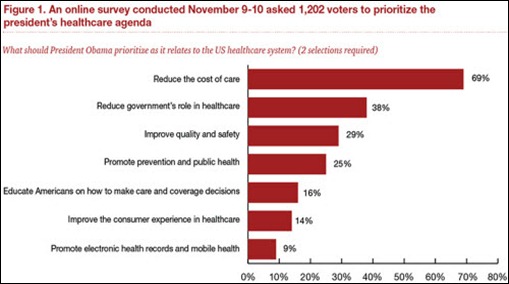
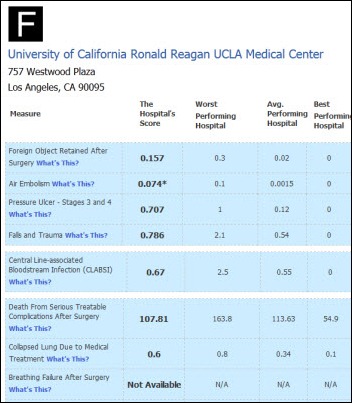

























I initially questioned the profile's authenticity because all of the headshots in the profile are clearly generated or enhanced by…