I've figured it out. At first I was confused but now all is clear. You see, we ARE running the…
Monday Morning Update 5/4/20
Top News
A Duke University-convened expert group lists short-term actions that can be taken to improve interoperability and data exchange for containing COVID-19. It recommends that:
- Commercial labs and point-of-care test manufacturers should record patient demographics along with COVID-19 samples and add the information to their reports to enable contract tracing, and CMS should use payment adjustments to give them incentive to do so. This information would include patient name, date of birth, gender, race/ethnicity, contact information (address and/or telephone number), and the identifier that was used in collecting the sample (such as medical record number). This capability could be brought online quickly by using the existing clinical query function of CommonWell, claims clearinghouses, or other information service providers.
- State and local health officials should define a minimum data set for COVID-19 containment as part of participating in clinical data exchanges. Limited public health resources precludes developing API-driven data feeds, so existing intermediaries should be used instead, such as Health Gorilla or the PULSE system that is supported by the Sequoia Project and Audacious Inquiry.
- Federal, state, and local officials should enhance their use of the National Syndromic Surveillance Program.
Reader Comments
From Marshall: “Re: Greenway Health. A rumored RIF of up to 10% of their workforce Thursday.” Unverified, but reported by several readers.
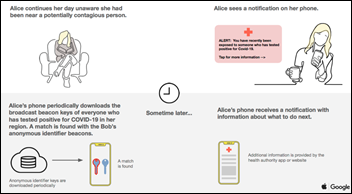
From Dill Fighter: “Re: Apple-Google contact tracing. I will address some misconceptions. The proposal mirrors the CDC in triggering notification only if you have spent at least 15 minutes within six feet of someone who is infected, so just walking by or checking out in a store won’t count. People won’t need to enable them since it will be built into the OS. Users don’t necessarily need to enter their own positive results – providers could enter them in a HIPAA-complaint way, such as entering only the Bluetooth ID of the phone.” My responses, adding to my initial comments:
- I missed the minimum time of contact specification, which according to Apple, requires 5-30 minutes of contact (the exact threshold must be defined by the public authority, which is responsible for analyzing the data). I assume that contact must be constant rather than cumulative.
- Apple and Google acknowledge that the six-foot range is a best guess based on Bluetooth signal strength and how the phone is being held. It will be thrown off if the phone is stored in a purse or backpack.
- Phone users don’t need to download the app, but they need to opt in when it is installed via an OS update. They can opt out or uninstall the app later, which may well happen if the app eats up battery power in the background.
- The user needs to manually install a second app that will be developed by their local public health authority (how that authority does that development isn’t clear). That authority sets the distancing rule and manages the entry of positive results, and without their app, the Apple-Google one does nothing. Apple and Google are suggesting that a future release of their app will eliminate the requirement of installing a public health app, although I haven’t seen a description of how that will work.
- It’s the user’s job to enter their own positive result using the public health service’s app. I haven’t seen any suggestion that the app will support providers doing it for them.
- Singapore saw barely more than single-digit adoption of its national contact tracing app. The country’s director of digital services, which developed the TraceTogether app, warns that they use it only to support manual contact tracing and it’s naive to see it as a replacement. He adds that “you cannot ‘big data’ your way out of a ‘no data’ situation,” such as the Washington state choir in which 45 of 60 members were infected despite distancing appropriately, likely because their singing projected respiratory particles further, and phone-based contact tracing would have missed that.
From HIMSSanity Cured: “Re: HIMSS. I don’t know about anyone else, but my consumption of HIMSS products and services is, and always has been, zero other than attending the conference.” Same here – I have no touch points with HIMSS other than the conference. I don’t read its publications, watch its webinars, attend its other events or local chapter meetings, pay for its certifications, follow its twitterati, view its endless ads, or participate in its plea for vendor-enriching government handouts (excuse me, “advocacy.”) I don’t say this as some kind of vindictive reaction to HIMSS policies and actions – I just don’t need anything that HIMSS offers and I don’t even think about the organization until it’s time to sign up for the conference (or not, as the case increasingly may be). They are just another vendor who I might contact in the unlikely event that I need something they offer. That’s just my opinion as a member, although even as a member I can’t say I’m thrilled at a lot of what HIMSS undertakes that seems more appropriate for a vendor than a member organization.
From Audioslave: “Re: podcast. Here’s a good one on public health.” I don’t listen to podcasts or watch videos that could have been presented as written articles instead. I know people have fun screwing around with their microphones and recorded video calls instead of writing, but they’re wasting my time to save theirs. I’ve done a zillion interviews and can say with confidence that skimming one in 45 seconds and reading the interesting parts more carefully is a lot more efficient than listening to a 30-minute conversation, especially when the questioner’s vanity prattling eats up an unreasonably high percentage of total run time.
HIStalk Announcements and Requests

A surprisingly large number of poll respondents have experienced some form of virtual visit since the pandemic broke out 100 years ago in mid-March, with video visits leading the pack. A couple of folks said that the video interface failed and the fallback was a phone call, while one also questioned the how good of replacement those visits can be when they offer only conversation and observation without the clinician being able to use a stethoscope or hands-on techniques.
New poll to your right or here: Will you use the COVID-19 contact tracing app from Apple and Google as soon as it becomes available?

I wonder if masks could be made from a clear but comfortable fabric so we don’t all wander around in public looking like bank robbers in Westerns? If not, here’s my Plan B: custom-printed masks where buyers can insert a headshot so that the outside of the mask looks like what is underneath (hello, CVS and Walgreens photo departments). We “Arrested Development” fans will be celebrating Cinco de Cuatro on Monday, so I confess that I was inspired by George Michael’s muscle shirt.
Webinars
None scheduled soon. Previous webinars are on our YouTube channel. Contact Lorre to present your own.
Acquisitions, Funding, Business, and Stock

Meditech reports Q1 results: revenue up 24%, EPS –$0.69 vs. $0.97, swinging from a quarter-over-quarter profit of $36 million to a loss of $26 million due to COVID-related stock losses and a decline in product bookings. Product revenue rose 77% and service revenue was up 3% in a quarter that was good for the company in the health IT market, but not so good in the stock market.
Spok reports Q1 results: revenue down 11%, EPS –$0.24 vs. $.04.
Government and Politics

President Trump nominates a replacement for the HHS principal deputy inspector general who interviewed hospitals about their COVID-19 concerns and reported their experience with shortages of coronavirus-related supplies and diagnostic tests. The President accused HHS career official Christi Grimm, MPA, who started working for OIG in 1999, of being politically motivated. The President has nominated as her replacement Jason Weida, JD, an assistant US attorney in Boston.
COVID-19
CMS issues another round of COVID-19 regulatory waivers that include:
- Physical therapists, occupational therapists, and speech language pathologists can provide Medicare telehealth services.
- Hospitals can bill Medicare for services that are provided remotely by hospital-based practitioners.
- Evaluation and management services can be delivered to Medicare patients via telephone.
- Behavioral health and patient education services can be provided by telephone and will be paid at the same rate as for office and outpatient visits.
- Medicare will pay for COVID-19 tests that are ordered by any healthcare professional, not just a physician, who is authorized by state law.
- Pharmacies can operate pharmacist-staffed drive-through testing sites if they are enrolled by Medicare as a laboratory.
- Hospitals will be paid separately for performing COVID-19 testing as the only service to a particular patient.
- Medicare and Medicaid will pay for certain FDA-authorized serology tests.
- Hospitals can increase COVID-19 beds without reducing their payments for indirect medical education, while inpatient psychiatric and rehabilitation hospitals can admit more patients without reducing their teaching payments.
- Hospitals will be paid at OPPS rates for outpatient services such as wound care, drug administration, and behavioral health that are delivered in temporary expansion locations, such as parking lot tents, converted hotels, and patient homes.
- Long-term acute-care hospitals will be paid at higher Medicare payment rates for accepting acute-care hospital patients.
- Nurse practitioners, clinical nurse specialists, and physician assistants can order home health services, establish and review plans of care for home health patients, an certify and re-certify patients for home health services.
- Physical and occupational therapists can delegate outpatient maintenance services to assistants.
- Applications for new ACOs will not be accepted until 2021, but those whose participation is expiring this year can extend for another year.
Analysis of TriNetX’s global health research network finds that patients aged 30 to 50 make up 26% of all strokes among patients who tested COVID-positive, versus the typical rate of 11% in non-infected patients in that age group.

The US is betting big ($483 million) that a coronavirus vaccine can be developed by messenger RNA drug company Moderna, which has never brought a product to market, hasn’t had any of its nine vaccine candidates approved by the FDA, and has never had a product reach the third phase of clinical trials. Even the company’s former chief science officer / R&D president is shocked by the huge amount of funding the government is providing. Nature magazine criticized the company for having failed to publish a single peer-reviewed paper about products it was touting to investors, likening it to Theranos. Moderna’s market cap has risen to $16 billion.
FDA gives Gilead emergency use authorization to distribute remdesivir for severely ill COVID-19 patients, also allowing five-day use for non-intubated patients instead of the usual 10 days, which will extend the drug’s supply. Gilead is donating its entire inventory of the drug, 1.5 million vials, to the federal government, which will oversee its distribution.
New research indicates that blood pressure drugs in the ACE inhibitor category, contrary to early concerns, do not affect coronavirus infection or outcomes.
A New York City nursing home admits that 98 residents of the 705-bed facility have died from presumed coronavirus infection.
A Brigham & Women’s ED doctor warns that it’s not reasonable to compare deaths from COVID-19 versus influenza – COVID-19 deaths count only patients who tested positive or met specific diagnostic criteria, while flu deaths are estimated using a model that adjusts for assumed vast underreporting (I admit that I did not know this). Example: CDC estimate 2018-29 US flu deaths at 26,000 to 53,000 even though just 7,200 deaths were confirmed. Applying that same underreporting assumption to COVID-19 suggests that it could have already killed 600,000 people in America (versus the official count of 68,000), and even then we are early into a pandemic that may or may not weaken in the summer.
Former FDA Commissioner Scott Gottlieb says that we may hit 100,000 US deaths from COVID-19 by June and that cases are still rising in 20 states, indicating that mitigation steps didn’t work as well as expected.
Meanwhile, the number of confirmed cases seems to have hit a stubborn plateau, leading to the possibility that a “second wave” won’t happen in the winter because the first one won’t actually have ended by then, especially with relaxed mitigation measures that the virus has waited out (late May is likely the new March as the April mitigation indiscretions kick in as active infections and hospitalizations). Seasonality remains the best (but uncertain) hope for a summer break. Curve-flattening was successful only in extending the time period in which the same number of people get infected, are hospitalized, and die, but otherwise the virus is still out there just like before.
China’s state media creates a video that makes fun of the US’s coronavirus response. Meanwhile, a Department of Homeland Security report says China intentionally hid the extent of the pandemic so it could hoard drugs and supplies, as evidenced by unusual import and export numbers.
Other
Epic launches Epic Health Research Network, a public-facing site in which Epic’s customers can post their observational findings about COVID-19 or any other topical issues in health and public health.
UK Prime Minister Boris Johnson and his fiancé give their newborn son the middle name of Nicholas in honor of two doctors by that name who treated Johnson for COVID-19 last month.
Sponsor Updates
- Clinical Computer Systems, developer of the Obix Perinatal Data System, releases the latest edition of its Critical Care Obstetrics Podcast, “Indications for Intubation.”
- OpenText’s information management solutions are now available as fully managed services on AWS.
Blog Posts
- Why Health Information Exchanges are an Important Pandemic Response Solution (Lightbeam Health Solutions)
- No Time for Training? No Problem! Georgia Health System Dives into LiveProcess to Manage COVID-19 Response (LiveProcess)
- Healthcare’s Economic Recovery will Depend on Rapid Adoption of RCM Innovation (Loyale Healthcare)
- Virtual care delivers on top priority for patient experience – safety (Meditech)
- Population Health Management: A Path to Value (Health Catalyst)
- Video Teleconferencing Security Tips for Zoom (Impact Advisors)
- 7 Essential Communication Tips When Reopening or Ramping Back Up Your Physical Therapy Practice (MWTherapy)
- Presenting Healthcare Heroes Their Capes on National Superhero Day (Netsmart)
- Preparing for “after COVID-19” – how can healthcare providers get ready for the pandemic’s re-scheduling aftermath? (Experian Health)
- How to Streamline the Patient Payment Collections Process When Staff is Stretched Thin (PatientBond)
- How to Measure Inter-professional Collaboration in Healthcare (PatientKeeper)
- 5 FAQs healthcare organizations are asking us about their FCC COVID-19 Telehealth Funding application (Pivot Point Consulting)
- Is your medical practice disaster and emergency proof? (PerfectServe)
- Contingency Planning During a Pandemic (Redox)
- Patient Engagement is Key to Telehealth Success (Relatient)
- Remote is the new normal: Enable workers, not hackers (SailPoint)
- Spirion Launches Program to Aid Local Restaurant Workers Impacted by COVID-19 Statewide Shut Down (Spirion)
- How to Handle Patient Moves and Bed Transfers; A17 HL7 Messages (Summit Healthcare)
- Safer, Hands-Free Communication for COVID-19 Environments – Deployed with Startling Speed (Vocera)
- 2 ways to support your payers through the COVID-19 crisis (Waystar)
- Envisioning a Better Normal: 3 Strategies to Prepare Your Practice for a Stronger Future (WebPT)
Contacts
Mr. H, Lorre, Jenn, Dr. Jayne.
Get HIStalk updates.
Send news or rumors.
Contact us.





Re: Under-reporting of COVID-19 deaths vs. Under-reporting of flu deaths
It seems that the under-reporting for COVID-19 deaths was similar to that of the flu early in the pandemic. A new study from Yale shows about 15,400 more deaths between March 1 and April 4.
https://www.vox.com/coronavirus-covid19/2020/4/29/21240393/voxcare-us-coronavirus-covid-19-deaths-how-many-yale
Re: clear masks — no thank you. Same with uncanny-valley “headshot” masks — nightmare fuel. Yikes. I am enjoying the creativity and personalization of masks in my area (I live in an area with a high density of both creative types and grandmas so lots of sewing going on around here) not to mention a high volume of gentlemenfolk running around looking like highwaymen with scarves and bandanas wrapped around their faces. Stand and deliver! (Adam Ant, your time is now.)