EPtalk by Dr. Jayne 6/24/21
In follow up to this year’s changes to the Evaluation & Management coding requirements, the American Medical Association announces clarifications that will hopefully make the codes easier to implement. The technical corrections updates are supposed to “add clarity and answer lingering questions.” The code updates were originally designed to reduce administrative burden on physicians while making it easier to document, although many organizations still have their providers hunting for bullet points because they haven’t made the required educational efforts to ensure everyone is on board with the changes.
The technical corrections include clarification on what constitutes “major” and “minor” surgeries as well as refinement of the meaning of “discussion” between physicians and other members of the care team, adding texts and instant messaging as methods as long as the process is interactive. It also further defined the meaning of “analyzed.” At this point, the corrections only apply to codes for outpatient or office settings.
For providers who are terrified of coding audits, anything that adds clarity is certainly welcome. My former employer took E&M coding completely out of the hands of providers, locking us out of the coding screens and shifting the work to coders. Although skilled, they were not certified professional coders, so the idea that charges were going out without my review always made me a little uncomfortable and was one of the reasons leading up to my departure.
The VA’s Cerner EHR modernization project is poised to receive an additional $56 million in budgeted funds for the 2022 program year. The additional funds are slated to support implementation at additional medical centers as well as to support infrastructure upgrades. According to a May report by the Office of the Inspector General, the VA’s facilities need electrical work, HVAC upgrades, and additional network cabling. More than two thirds of the VA’s medical centers are over 50 years old, with the average age being 58.
I’m always excited to learn about how technology is impacting public health, so I enjoyed reading a recent JAMA Surgery article about the “Association of Rideshare Use With Alcohol-Associated Motor Vehicle Crash Trauma.” The authors set out to determine whether use of rideshare services decreased impaired driving, resulting in changes to motor vehicle trauma rates. They looked at data from the Houston, TX metropolitan area that included hospital data and court records on convictions for impaired driving, along with rideshare data from Uber and Google. They found that “rideshare volume had a significant negative correlation with the incidence of motor vehicle-associated trauma, and this was most evident in those younger than 30 years; a significant decrease in convictions for impaired driving was associated with the introduction of rideshare services.” That’s fantastic news for those of us who have ever had to staff a trauma bay.

I thought summer was going to be a slow time for me, but I’ve picked up some projects that are going to take a lot more of my time than I thought. Based on some of the slowdowns in 2020, I’m happy to have the work and even happier that organizations feel stable enough to go back and work on projects that were truncated or even canceled by the pandemic. Some of the things I’m working on include vaccination campaigns, chronic disease outreach, and cancer screening campaigns. (As far as colorectal cancer screening is concerned, did you know that 45 is the new 50? If you’re 45 or older, it’s time to consider a colonoscopy or stool testing.) These are the bread-and-butter kinds of initiatives that I wish more organizations would work on. I’m working with a handful of patient engagement solutions across my clients and it has been an interesting exercise to compare their capabilities.
I was glad to enjoy some blue skies recently, though, and would encourage everyone to find time to just let your brain turn off. Or, if you’re not into just sitting around watching crops grow, consider reading a book just for enjoyment or hanging out with friends you haven’t connected with in a while, even if it has to be virtual. Many of us have been working hard over the last year and a half that COVID-19 has been with us and it’s time to recharge our batteries. Although I’m very confident in the performance of vaccines against the virus as we know it now, it feels like it’s only a matter of time before some kind of other proverbial shoe drops. When it does, I want to be rejuvenated and ready for action. I’ll be taking a couple of days to do some rock climbing and other adventures and to continue to reset my brain and to get ready for whatever gets thrown at me next.
HIMSS is approaching and I received my first emails this week, asking if I was interested in scheduling meetings. Both vendors were ones I hadn’t heard of, so maybe the “new normal” HIMSS is an opportunity for smaller companies to share their messages without being buried in the noise. One of the vendors has a lot to learn about email marketing – other than the “schedule a meeting” link, nothing in the email was dynamic. I couldn’t even click on a company logo to go to a website and learn more about what the company does. The scheduling link at least took me to a part of the company’s website where I could tour, but I still don’t fully understand what they do.
In talking with some of my usual HIMSS buddies, one is moving to the Caribbean so will not attend, one is putting their final plans together, and the other is onboard with planning our annual booth crawl. I am still curious what the social scene will look like and whether there will be off-campus events with food and beverage offerings or whether most vendors are doing the Wednesday afternoon exhibit hall happy hour.
Have intel on the HIMSS social scene? Leave a comment or email me.

Email Dr. Jayne.




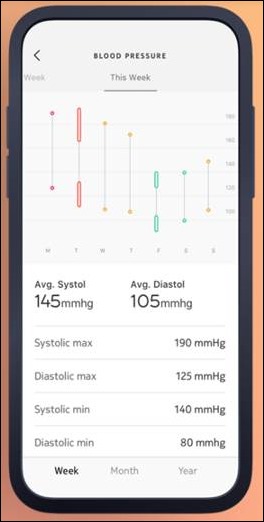


















I've figured it out. At first I was confused but now all is clear. You see, we ARE running the…