Healthcare AI News 10/9/24
News
WVU Medicine implements an AI-powered search system that allows employees to search for content that is contained in internal documents, such as clinical protocols, how-to guides, and tip sheets. It plans to eventually offer searching across all of its sites as well as across all Epic-using hospitals.
A newly signed California bill requires providers to include a specifically formatted disclaimer when AI was used in patient-facing clinical communication that has not been reviewed by a licensed provider. The communication must also contain instructions for the patient to contact an appropriate person.
HHS Assistant Technology for Technology Policy and AI leader Micky Tripathi, PhD, MPP says that HHS is looking at ways to create AI models using its vast data stores, but he cautions that its data is inherently biased since it excludes people who don’t have insurance or who don’t seek care from providers.
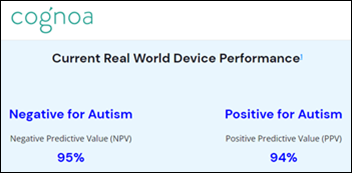
Wyoming’s Medicaid program approves coverage of Canvas DX, an online tool that assesses autism using family-provided information and videos. Families can use the tool instead of trying to find one of the few state clinicians that can administer the ADOS test.
The American College of Radiology will participate in the FDA’s program to boost innovation in breakthrough devices. FDA has expanded the program for its cardiology device origins to include neurology, ophthalmology, and radiology.
Business

The authors of a literature review on AI in healthcare suggest that its public health impact will be limited by the “politics of avoidance,” where the US health system’s treatment-focused model ignores broader social determinants of health. They express concern that the hype around AI may overshadow more effective, evidence-based interventions like using community health workers and implementing harm reduction programs, which address root causes rather than just offering suggestions for treating symptoms.
Research
A test of GPT-4’s ability to provide clinical recommendations for ED visits – including admission status, radiology requests, and antibiotic prescriptions – finds that it performs poorly compared to medical residents. Its recommendations were often overly cautious and called for medically unnecessary admissions and orders.
A review of AI-powered, FDA-approved medical devices finds that most did not include race or ethnicity, socioeconomic data, and the ages of study participants in their submitted approval documentation. The authors conclude that the lack of consistency and data transparency may exacerbate health disparities
Other

Drug maker Eli Lilly and Company names Thomas Fuchs, DrSc as its inaugural chief AI officer, where he will be tasked with leading the company’s AI initiatives in drug discovery, clinical trials, and manufacturing. He was previously chair of the AI program at Icahn School of Medicine at Mount Sinai.
Mayo Clinic will use a $25 million donation to provide funding and work time for its clinicians to purse AI-related projects.
Four high-profile cancer centers use $40 million in tech company funding to form the Cancer AI Alliance, which will study their collective patient data to find clinical insights.

US Army researchers develop SeptiBurnAlert, which uses AI to analyze the blood samples of burn patients to detect sepsis-related components. The system predicts risk within the first 24 hours of hospitalization with high accuracy. The team has applied for a patent and hopes to bring the product to the commercial market in three years, pending FDA approval. They are also working with companies that want to product a handheld device.
Contacts
Mr. H, Lorre, Jenn, Dr. Jayne.
Get HIStalk updates.
Send news or rumors.
Contact us.



Today's post contains the phoenixes rising from the ashes of the post COVID telehealth era. There's two things that destroy…