It's nice when Epic takes on patent trolls and other bad actors in the industry. They do great when they…
Monday Morning Update 8/31/20
Top News
Konica Minolta will pay $500,000 to settle false claims act charges involving its former subsidiary Viztek, which the federal government says fraudulently obtained certification for its Exa EHR that allowed users to falsely collect Meaningful Use payments.
Also named was EHR certification company InfoGard, which “facilitated and participated” in Viztek’s false attestations by certifying the product even as its tester noted obvious manipulation.
Details of the whistleblower’s complaint, in which the government intervened without filing its own complaint, are more interesting than the $500,000 settlement might suggest:
- PACS vendor Viztek announced rollout of its EHR-PACS integrated Exa EHR in mid-2014. It developed that product in reworking its previously acquired Opal EHR, which was ONC certified.
- Viztek’s India-based developers underestimated the work that was required to bring Exa EHR up to 2014 edition standards.
- The whistleblower – Exa EHR’s product manager – said Viztek founder and president Joe Cermin told her that “I don’t care if you have to lie, beg, cheat, steal, or kill” to earn certification since failure to do so would jeopardize the millions of dollars that would result from the company’s acquisition by Konica Minolta, which was underway during the certification testing.
- Konica Minolta acquired the 120-employee, North Carolina-based Viztek in October 2015.
- Viztek chose the remote testing option so it could manipulate the testing scenarios using a hard-coded product version that was never released. The whistleblower was told to keep multiple tabs open on her screen, one to run the test script and the other to show an already-configured result. The software failed testing at several points, at which time the company’s executives would ask for a break to allow two on-call teams in India to dummy up test results on two versions of the software, then demonstrate the result of whichever team finished first. At several points, the developers accidently displayed live patient data.
- The developers hard-coded the EHR to pass the XML output requirements for Common MU Data Set by using programming they found on an ONC testing website. They didn’t even bother to remove the other EHR vendor’s name that was still embedded in the programming.
- InfoGard “facilitated and participated” in the false attestations by giving Viztek multiple attempts to pass and approving frequent breaks and delays that gave developers time to falsify the programing. The InfoGard tester passed the product even though she noticed that on-screen version numbers, colors, and field layouts changed after the developers had taken breaks.
- The UL subsidiary of Underwriters Laboratories acquired InfoGard in December 2015.
Reader Comments
From Debbie Downer: “Re: [health IT executive name omitted.] Does it seem they had to be at least somewhat evil to have made their way to the top?” We all have a good-bad behavior ratio that changes situationally, earning us a perceived “jerk score” that may be based on only superficial aspects of our character as observed at our most inopportune moments. Steve Jobs, Bill Gates, Elon Musk, and Neal Patterson kicked their dents in the universe with a psychologically obsessive focus, notoriously flaring temper, an intolerance of naysayers, psychological issues that in some cases were tied to a traumatic childhood, and a general indifference to the wellbeing of the folks who were rowing their corporate boat. But they built lasting and daringly innovative companies in their image that likely would not have happened if they were easygoing everymen. It’s probably not true that nice people finish last, but it is true that people who are successful in any field have to push themselves and others in ways that cheap-seaters would likely find despicable. Whether that leaves them happier on their deathbeds or whether society is better off as a result is an issue that is above my pay grade. Maybe my conclusion is that you can be happy only if you act as your natural self, regardless of how acceptable that may or may not be to others, and the vast majority of jerks don’t accomplish all that much.
From Woodstock Generation: “Re: US withdrawal from WHO. All of my HIM colleagues say no impact will result. We will continue to use ICD-10 diagnoses from WHO and modify them as usual to crate our ICD-10 CM. We will also will continue to create procedure codes (ICD-10 PCS) for use only in the US.”
HIStalk Announcements and Requests
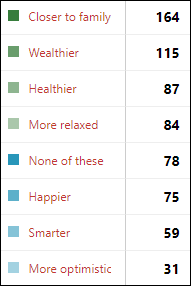
Many poll respondents say that their family connection and money situations have improved in the past year, although quite a few others don’t have much positive to report (with “optimism” taking a big hit, likely due to COVID-19).
New poll to your right or here: how much consumer and healthcare impact will the Amazon Halo wearable have?
Webinars
September 3 (Thursday) 2 ET. “How Does A Global Pandemic Reshape Health IT? A Panel Discussion.” Sponsor: Intelligent Medical Objects. Presenters: Rob Wallace, chief product officer, IMO; Andrew S. Kanter, MD, MPH, chief medical officer, IMO; Lori Kevin, VP of enterprise IT and security, IMO; Sahas Subramanian, MCA, enterprise architect, IMO. As COVID-19 continues to spread, regulation changes, code system updates, and an increased reliance on technology are making it hard to stay on top of the many ways the pandemic is altering health IT. What’s more, we’re confronting challenges that rely heavily on technological solutions – like accurate reporting tools or telehealth adaptations – and we need those solutions now. The panel of subject matter experts across the enterprise will share insights on how the global pandemic is reshaping the health IT world.
September 17 (Thursday) 1 ET. “ICD-10-CM 2021 Updates and Regulatory Readiness.“ Sponsor: Intelligent Medical Objects. Presenters: June Bronnert, MSHI, RHIA, VP of global clinical services, IMO; Theresa Rihanek, MHA, RHIA, mapping manager, IMO; Julie Glasgow, MD, clinical terminologist, IMO. IMO’s top coding professionals and thought leaders will review additions, deletions, and other revisions to the 2020 ICD-10-CM code set that will be critical in coding accurately for proper reimbursement.
Previous webinars are on our YouTube channel. Contact Lorre to present your own.
Acquisitions, Funding, Business, and Stock
Prescription shopping vendor GoodRx files for an IPO, showing first-half results of $55 million in profit on $257 million in revenue with high growth in both. The company was valued at $3 billion in a 2018 funding round.
COVID-19
US COVID-19 deaths are at 183,000, with 220,000 projected by November 1.
Public health experts question whether COVID-19 PCR tests are overly sensitive, causing people who are carrying insignificant amounts of virus to be labeled as positive and treated as contagious. The answer isn’t to stop testing, as CDC’s controversial new guidance suggests, but instead to use the newly introduced rapid tests that are less sensitive. Other options would be to confirm PCR tests a few hours later with a rapid test, or to interpret the same PCR test result using lower cycle threshold ranges. A run of samples at New York’s state lab identified 794 positive results using the common setting of 40 cycles, but detuning the sensitivity to 35 cycles reduced that number in half, which would make the results more meaningful and make contact tracing easier. The experts also question why labs report their test results as simply positive or negative instead of listing the actual measured viral load.
HHS dismisses two of its high-level PR experts following the backlash that followed erroneous statements made by FDA Commissioner Stephen Hahn, MD about the effectiveness of convalescent plasma treatment that he later declined to correct in a public forum. Hahn, President Trump, and HHS Secretary Alex Azar touted FDA’s Emergency Use Authorization of the treatment as a “very historic breakthrough” that offers a 35% reduction in deaths, a wildly incorrect misinterpretation of results from an observational study that showed that a tiny subset of patients had 35% fewer deaths when given the treatment early versus those who were given it later. Hahn tweeted that he misspoke in characterizing the findings as an “absolute reduction” instead of a “relative reduction,” but he has not elaborated further or provided more accurate information to the public. No randomized clinical trial has been done to prove that convalescent plasma treatment reduces deaths, and even taking the data at face value suggests a 5% mortality reduction at best from using the 100-year-old treatment.
FDA assigns Emergency Use Authorization to allow all hospitalized COVID-19 patients to receive remdesivir, although no research has been published to prove its benefit. FDA issued an EUA in May for using the drug in non-ventilated hospitalized patients who need oxygen. FDA Commissioner Stephen Hahn, MD said that “data show that this treatment has the potential to help even more hospitalized patients,” citing one study from May and another that found only that five days of treatment work as well as 10. Hospitals worry that broader authorization is unproven, it came in the absence of new research, and the EUA will make it harder to obtain the drug for patients whose need is better documented by evidence.
FDA Commissioner Hahn says the agency will authorize widespread use of a COVID-19 vaccine via Emergency Use Authorization before Phase III clinical trials are complete if they think the benefits outweigh the risks. He says it won’t be a political decision – companies apply for such authorization and FDA makes a determination based on the evidence they submit. The only countries that have approved vaccines before their clinical trials were completed are China and Russia.
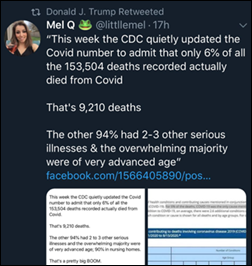
People who are misinterpreting CDC’s data are spreading the rumor that only 6% of reported coronavirus deaths were caused by COVID-19, confusing the fact that 6% have COVID-19 as the only ICD-10 code listed while the other 94% include COVID-19 as well as comorbidities such as obesity or diabetes, as is common in many Americans and nearly ubiquitous in older people. It was already widely published that older, sicker people are more likely to die of COVID-19, as are minorities and those who are poorer. The pandemic won’t just go away by pretending that people who die of pneumonia in conjunction with coronavirus infection didn’t really die of COVID-19 and therefore everybody else’s odds are better. But you want to blame people for letting COVID-19 kill them by daring to be older, sicker, poorer, or less white, then these are some good numbers to share with others who don’t really care about their deaths either.
Other
Ascension Michigan will lay off 223 employees of its IT network operations and service desk in October as the health system outsources their jobs. SVP/CIO Gerry Lewis said in a blog post two weeks ago that “we have begun shifting some of our technology functions to third-party partners who specialize in these services” as part of its “digital transformation.”
Cleveland Clinic President and CEO Tom Mihaljevic, MD will interview Epic CEO Judy Faulkner on Wednesday, September 2 at 6 p.m. ET as part of the speaker series “Ideas For Tomorrow.” It will be live-streamed on Facebook and YouTube Live.
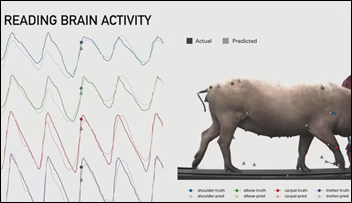
Elon Musk’s Neuralink demonstrates its skull-inserted brain-computer link in a pig, as the company continues its progress toward creating “neural shunts” that could allow paraplegics to regain use of their limbs. Musk also envisions people communicating using “conceptual telepathy” without writing or speaking. He admits that people will be wary of the technology that he calls “a Fitbit for your skull,” acknowledging that “this is increasingly sounding like a ‘Black Mirror’ episode.”
Sponsor Updates
- OptimizeRx CEO William Febbo will present at the LD 500 virtual investor conference on September 3.
- The National Council for Prescription Drug Programs honors Surescripts Clinical Informatics Manager Larry King with its Rising Star Award.
- TriNetX announces that Duke-NUS, a medical school in Singapore, has joined its global research network.
- Vocera will present virtually at the Baird 2020 Global Healthcare Conference September 9, Wells Fargo Virtual Healthcare Conference September 10, and Morgan Stanley Virtual Global Healthcare Conference September 14.
- Wolters Kluwer launches “5 Forces for the Future” series to reimagine healthcare post-COVID-19.
Blog Posts
- The difference between ambient clinical intelligence and ambition (Nuance)
- How Far We’ve Come: Immunizations (OmniSys)
- My Unexpected First 100+ Days (PatientKeeper)
- Clarifying 5 Misconceptions about CMS’s E-Notifications Condition of Participation (PatientPing)
- Investing in Partnerships Pays Dividends (PMD)
- Executive Analysis Elevates a Transfer Center’s ROI and Strategic Value (Central Logic)
- Take Advantage of the PAMA Deadline Extension (Premier)
- Sell to hospitals. Get your product live. (Redox)
- Back to the Future with SailPoint Predictive Identity (SailPoint)
- 3 key questions to determine if your data protection software is Privacy-Grade (Spirion)
- How to strengthen your emergency call system to comply with Kari’s Law and the Ray Baum’s Act (Spok)
- Vocera and EASE: Humanizing the Patient and Family Experience, Together (Vocera)
- What Healthcare Can Learn From Fintech’s Digital Evolution (Waystar)
- Is Professional Liability Insurance Worth It? (WebPT)
Contacts
Mr. H, Lorre, Jenn, Dr. Jayne.
Get HIStalk updates.
Send news or rumors.
Contact us.




WHO: WHO is not about ICD-10……It’s to be a non-political group (hence, UN), to pool resources to prevent containable and preventable health disparities. Let’s not go down rabbit holes to try to oversimplify it’s purpose to justify the despicable behavior of the US deactivating the CDC power, which is really why WHO was disabled.
Ms. Goss, the UN and WHO is political and I would remind you to read the statements of the WHO leaders in JAN & FEB, which absolved China of any responsibility for C-19, its development or being the “bad actors” that every health company knows they are from IT stealing, to outright lying.
“But they built lasting and daringly innovative companies in their image that likely would not have happened if they were easygoing everymen. It’s probably not true that nice people finish last, but it is true that people who are successful in any field have to push themselves and others in ways that cheap-seaters would likely find despicable.”
well said!
I find it interesting that the testing organization, (InfoGard) couldn’t ferret out hard coded data. It is really as simple as sampling from the top n (100) plus alpha and noticing that every time you change the value for n the source system has to go back and ‘redo’ their work. Or, asking the question: “Can you show me your dictionary for x in SQL?” then ask them to do a count of that dictionary and compare it to the expected value. If you are off by hundreds of thousands then you hard coded values.
By that I mean, RxNorm has 70k? values in the in/min and brand TTY? If the solution under test can’t show an RxNorm table, or its equivalent (Medispan, FirstData, Multum) with a pretty close proximity to that number then you have a Houston moment.
This isn’t rocket surgery, that pattern of ask/fail/return with the correct answer wouldn’t pass a grade school teacher’s closed book exam sniff test. Just as it should never have passed their tests.
We now have more than enough examples of this happening in the industry that the testing orgs should seriously consider what value they are adding to the problem space.
The industry as a whole should be looking at their Pareto usage items and asking for those items from their regional EHRs. 80% of the items transmitted will be from 20% of the dictionary (for some domains the ratio is different but still relevant) and if those 20% can’t be added, exchanged, reconciled and incorporated, and returned to the original source without change then that is a huge problem.
Unfortunately, there are plenty of business majors who have no regard for the lives under their client’s care. The old adage that a CEO makes the big bucks because they take all the risk has long fallen to the wayside.
Who’s watching the watchmen? I was led to believe ONC was responsible for oversight of these testing bodies, but that federal office appears committed to issuing rules/guidance rather than enforcing them.
It’s not that they couldn’t, they just aren’t paid for that. Infogard is paid to rubber-stamp certifications and treats some fraud fines as the cost of doing business.
I think the real story here is that HHS and the feds rely so heavily on private contractors and private industry. Teasing out why and enacting change is something not even on anybodies radar. 50 years ago the feds would have just done this certification work themselves and there wouldn’t be the same mismatched incentives. No it is outsourced three different ways, costs more, and still nothing gets done.